Processes and Requirements for Exporting Medical Devices to Indonesia
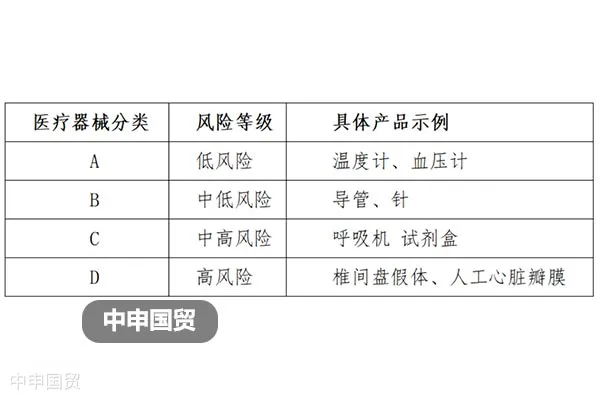
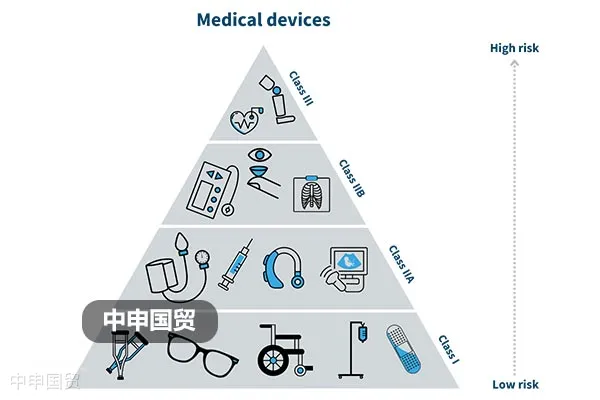
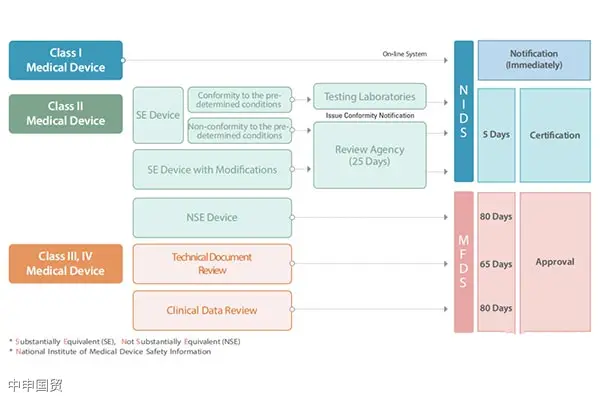
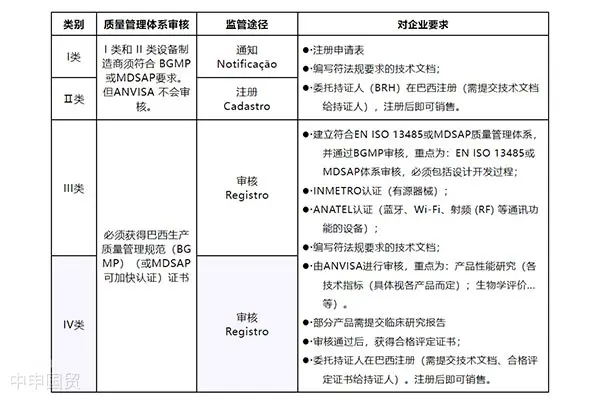
This article provides an in-depth exploration of the Indonesian medical device market, covering regulatory authorities, medical device classification, market registration processes, registration document requirements, and labeling regulations, aiming to offer reference and guidance for Chinese medical device companies to successfully enter the Indonesian market.










 PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912



