- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
On July 19, 2023, the European Commission madeCosmetics & Personal Caresignificant amendments to Regulation (EC) No 1223/2009, issuing the new Regulation (EU) 2023/1490. These amendments primarily target the List of Prohibited Substances in Cosmetics (Annex II), adding 30 substances considered carcinogenic, mutagenic, or toxic to reproduction (CMR).

Among the newly added prohibited substances, six ingredients are already included in the Inventory of Existing Cosmetic Ingredients in China 2021 Edition (IECIC 2021). This means these substances are widely used in Chinas cosmetics industry but will face strict restrictions in the EU in the future.
For Chinas cosmetics industry, the EUs regulatory amendments undoubtedly pose challenges. First, enterprises must inspect whether their products contain these soon-to-be-banned substances and make necessary adjustments before the regulation takes effect. This is to avoid failing product safety assessments, which could impact exports.
At the same time, we remind domestic enterprises to monitor changes in the EUs regulatory requirements for these ingredients. The EUs ban will not only affect Chinas export market but may also influence domestic cosmetics safety assessments. Special attention is needed for the six substances not yet banned in China.
Regulation (EU)2023/1490 will be inEffective from August 8, 2023[Export Key Points] Detailed Explanation of the Application Process for Chinas Export License!The implementation date is set for December 1, 2023.
Substances not yet banned domestically
The following substances are listed as prohibited by the EU but are not yet banned domestically (included in the Inventory of Existing Cosmetic Ingredients in China 2021 Edition (IECIC 2021)).
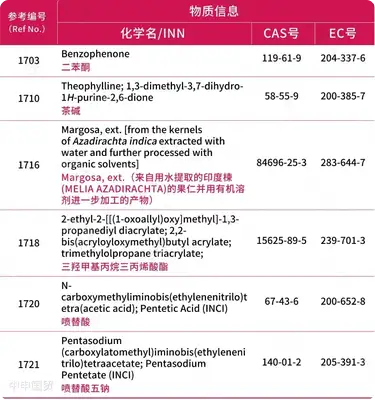
Content of the EUs Revised Cosmetics Regulation
Below is the full content of the EUs revised cosmetics regulation:
Modification of information for 1 substance
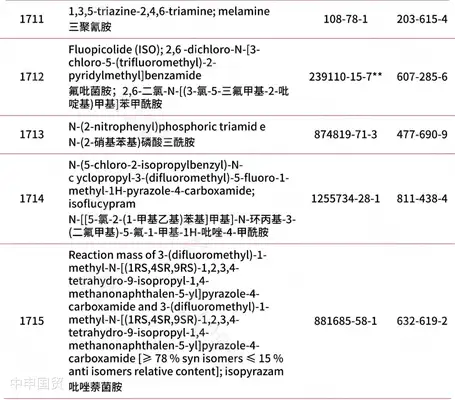
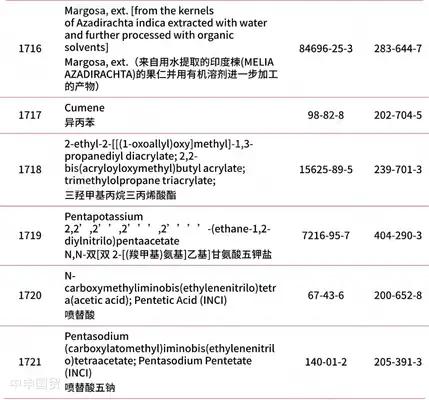
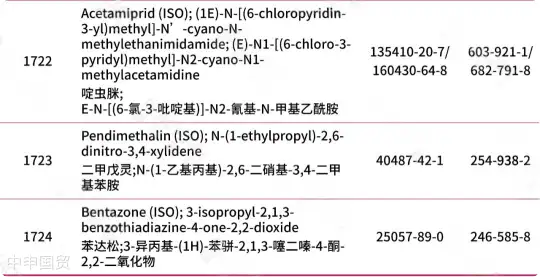
* Red text indicates newly added content.

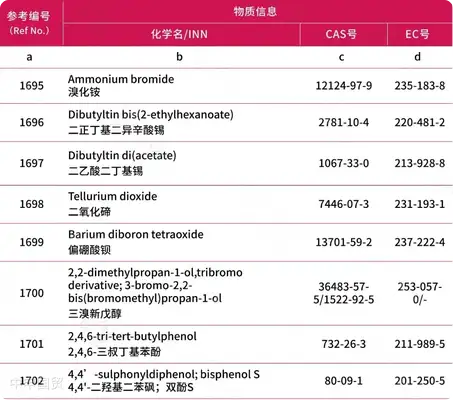
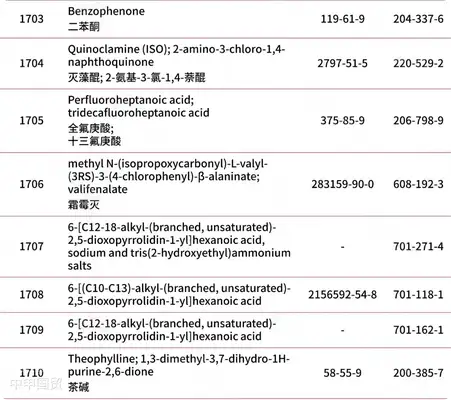
Addition of 30 prohibited substances
Related Recommendations
? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912